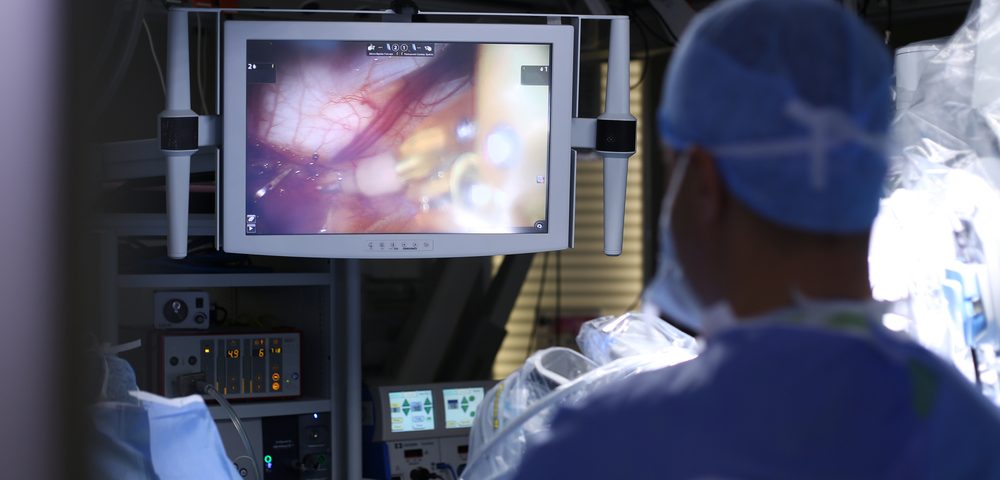
OTL38, a tumor-specific fluorescent compound that “lights up” ovarian tumor tissue during surgery, helped doctors to detect and remove cancer cells that would otherwise be missed, final results of a Phase 2 study show.
The study, “A phase II, multicenter, open-label trial of OTL38 injection for the intra-operative imaging of folate receptor-alpha positive ovarian cancer,” was published in the journal Gynecologic Oncology.
“OTL38 can intraoperatively identify more ovarian cancer lesions during … surgery than traditional detection techniques alone which enables a better outcome for patients,” Chris Barys, CEO of On Target Laboratories, which developed OTL38, said in a news release.
The positive data has led to a Phase 3 study (NCT03180307) currently evaluating the safety and effectiveness of OTL38, compared with current methods, in detecting additional ovarian cancer cells during surgery.
The complete removal of a tumor during surgery helps to avoid additional surgeries and cancer relapse, but, most importantly, to increase patients’ overall survival. Currently, the strongest predictor of overall survival is the removal of all macroscopic disease during surgery, where the doctor distinguishes cancer from healthy tissue by visual inspection and palpation.
Fluorescence-guided surgery, which combines imaging techniques and tumor-specific fluorescent tracers — fluorescent compounds that selectively bind to tumors — has the potential to provide highly specific real-time detection of tumors during surgery, illuminating cancer cells that once evaded standard detection.
OTL38, consisting of a folic acid molecule bound to a green-like near-infrared dye, is designed to bind to folate receptor alpha (FRa) in cancer cells and accumulate inside them. Cancer cells produce 10- to 100-fold more FRa than normal cells, making it a suitable target molecule to mark cancer cells.
The open-label, multicenter Phase 2 study (NCT02317705) evaluated the safety and efficacy of OTL38 in detecting FRa-positive ovarian cancer during surgery to remove the tumor.
The study involved 44 adult women (mean age of 64 years) with known or suspected ovarian cancer and a planned surgery to remove it. Most of them were white (79.5%) and had advanced or metastatic (70.3%), or high-grade serous (61.4%) ovarian cancer.
OTL38 was injected into the cancer region two to three hours before surgery. A total of 29 women (60.4%) underwent fluorescence imaging during surgery and had at least one FRa-positive ovarian tumor-like lesion. While safety was evaluated for all women, effectiveness was assessed considering only the data of these 29 women.
Surgeons collected a total of 225 lesions from these women, including 171 lesions that “lit up” and were FRa-positive ovarian cancer lesions (true positives), 23 lesions that fluoresced but were not positive for FRa or cancer (false positives), 28 lesions that did not light up but were positive for FRa and cancer (false negatives), and three lesions that were not cancerous and did not fluoresce (true negatives).
Overall, when accounting for a possible association among multiple cancer lesions within a single patient, 98% of the lesions “lit up” with OTL38, and 95% of the illuminated tissue was cancerous.
OTL38 showed comparable efficacing in all lab-confirmed ovarian cancer lesions (whether FRa positive or negative), suggesting that it may be used even in patients with unknown FRa status or FRa-negative cancer.
The team noted that since most OTL38-false positives were located in the lymph nodes, further research is needed to confirm and characterize these false positives.
Also, surgeons were able to detect at least one additional tumor-like lesion in 14 women (48.3%), and four or more additional lesions in 40% of the women with miliary disease (widespread, millet-like lesions), with OTL38 imaging alone.
Eight patients had mild OTL38-associated adverse events, including local skin reaction to OTL38 injection, nausea, vomiting, and abdominal pain. These gastrointestinal-related adverse effects might be associated with “the typical disease distribution of ovarian cancer, [and] miliary disease deposits involving the gastrointestinal tract,” the researchers wrote.
They also emphasized that OTL38-associated toxicity was mild and disappeared within 24 hours after OTL38 administration.
The results suggested that OTL38-guided surgery was safe and allowed the detection of additional ovarian cancer lesions that would otherwise be left behind without the use of OTL38.
“Using such a fluorescent imaging [tracer] with high affinity and specificity to ovarian cancer helps surgeons to more effectively remove additional disease, potentially impacting patient survival outcomes,” said Janos Tanyi, MD, PhD, the study’s senior author.
OTL38’s Phase 3 study (NCT03180307), which is still recruiting, is expected to enroll 170 women with ovarian cancer at 10 sites in the U.S. and one in the Netherlands.
Other clinical trials are currently or will be evaluating OTL38’s safety and effectiveness in people with lung cancer and inflammatory diseases, including rheumatoid arthritis and inflammatory bowel disease.