Patients with recurrent ovarian cancer may greatly benefit from the addition of Tumor Treating Fields (TTFields), a method that uses electric fields to disrupt cell division, to Taxol (paclitaxel), according to recent data from the pilot INNOVATE trial.
Recent data presented at the American Association for Cancer Research (AACR) 2017 Annual Meeting in Washington, D.C., has shown that the combination more than doubled patients’ median progression-free survival (PFS) compared to historical controls receiving Taxol alone. The study was titled “INNOVATE: a phase II study of TTFields (200 kHz) concomitant with weekly paclitaxel for recurrent ovarian carcinoma.”
“A clear unmet need remains for patients with recurrent ovarian cancer, particularly in the platinum-resistant population, with median overall survival of only 13 to 14 months post recurrence,” Dr. Igance Vergote, chair of the Department of Obstetrics and Gynecology at Catholic University Leuven in Belgium, said in a press release. “These data show that treatment with TTFields has the potential to make a difference in the lives of recurrent ovarian cancer patients.”
Novocure‘s TTFields is a noninvasive, regional treatment modality that is currently approved by the U.S. Food and Drug Administration to treat patients with newly diagnosed glioblastoma, an aggressive type of brain cancer.
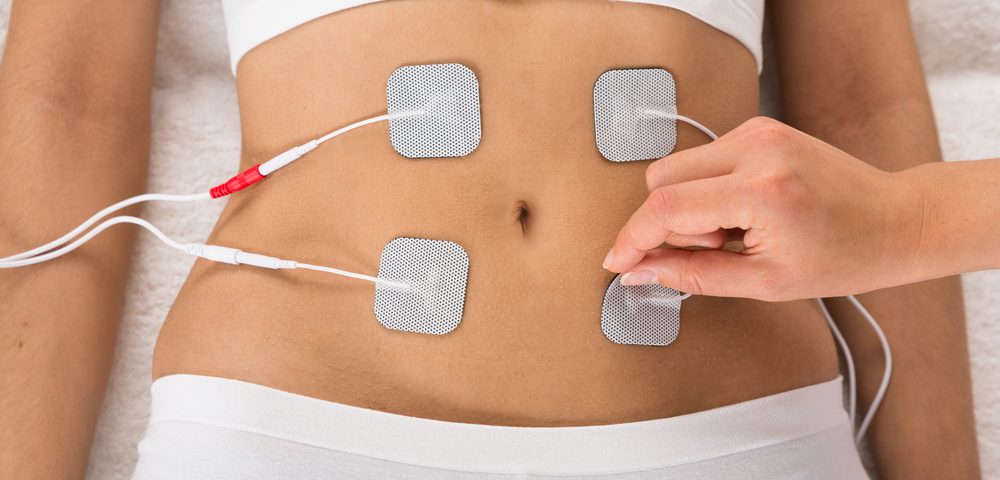
It acts by delivering intermediate frequency alternating electric fields to the tumor, which activate programmed cell death in proliferating cancer cells by preventing them from dividing properly. The system works through the application of four electrodes on a woman’s torso in the region surrounding the tumor. The delivery system is portable and allows patients to maintain a regular daily routine.
The Phase 2 INNOVATE trial (NCT02244502) was the first study testing TTFields (200 KHz) in ovarian cancer patients. The study enrolled 31 patients who were treated continuously with TTFields — using the NovoTTF-100L(O) device — and weekly Taxol (80 mg/m²) for eight weeks, followed by treatment at days 1, 8, and 15 of each subsequent 28-day cycle.
Results have shown that patients’ median progression free survival was 8.9 months, which more than doubled the 3.9 months seen in historical controls treated with Taxol alone.
Median overall survival had not been reached at the time of the analysis, but 61% of patients were still alive one year after starting the treatment. This suggests an improvement over the 13 to 14 months median overall survival reported in historical controls.
The researchers also reported a median compliance of patients receiving TTFields of 77% in the first three months.
Apart from two cases of severe skin irritation, no other serious device-related adverse events were seen.
“These are encouraging results in a disease state that is very difficult to treat and we are now working to develop a Phase 3 pivotal trial protocol to further study TTFields for the treatment of recurrent ovarian cancer,” said Dr. Eilon Kirson, Novocure’s chief science officer and head of Research and Development.
“These data give us hope that TTFields used in combination with other cancer treatments may increase survival without significantly increasing side effects for a variety of solid tumors,” Kirson said.